Basic Hydrolysis of polymers
The hydrolysis of polymers involves a chemical reaction in which water interacts directly with the polymer. This interaction breaks the bonds that hold it together, and reduces the polymer into monomers or smaller polymer chains called oligomers. This is the opposite of polymerization – the process that creates polymers by combining monomers.
The word “hydrolysis” which means separation, comes from the Greek words “hydro”, meaning water. Hydrolysis occurs when water molecules break or lyse the bonds in a molecule.
Hydrolysis is a primary cause of degradation for many polymers. This includes those that are used in wet or watery environments. It can affect the mechanical and physical properties of polymers as well as their lifespan. These polymers are prone to hydrolysis because they have ester groups, amides, or urethanes.
See more about What is hydrolysis
As an example, the hydrolysis reaction of polyester could be described as follows:
[ -O-CO-R- ]n + nH2O – nHO-R + nCO2
The R on the right side could represent any organic group. This can vary depending on the type of polyester.
Urethane hydrolysis and degradation
Hydrolysis can cause polyurethane or urethane to degrade, especially in high-temperature and hi humidity environments. This hydrolysis breaks the urethane bond and reduces mechanical strength. Long-term exposure to UV radiation may cause color loss and reduced elasticity. Thermal degradation can be caused by high temperatures, which alters the polymer’s structure and causes discoloration. PU can be further damaged by oxidation, chemical attacks, and mechanical stress. Protective coatings and additives can be used to reduce degradation. Or, more stable types of polyurethane could be chosen based on intended environment.
Hydrolysis Test of PU
Hydrolysis tests for Polyurethanes are conducted by simulating an environment that is moist to test the durability of the material. The PU samples will be placed in a controlled environment with high humidity and temperature for a specified period of time to simulate accelerated aging. Tests are performed to determine if there have been any changes in the material’s properties, such as elasticity and visible surface modifications. The results are compared with the original properties of the material in order to determine the PU’s hydrolysis resistance. This information can be used to determine if a PU is suitable for use in a specific environment or if it needs improvement.
Use of Carbodiimide as an Anti-Hydrolysis agent in Polyester Polyurethane
As anti-hydrolysis agents, carbodiimides are effective in polyester polyurethane. This compound reacts with the water-sensitive ester bonds within the structure of polyester polyurethane. Carbodiimide breaks these bonds first instead of the water molecules, which happens during hydrolytic degradation. Carbodiimide is a great way to improve the durability and performance of polyester polyurethanes in wet or humid environments.
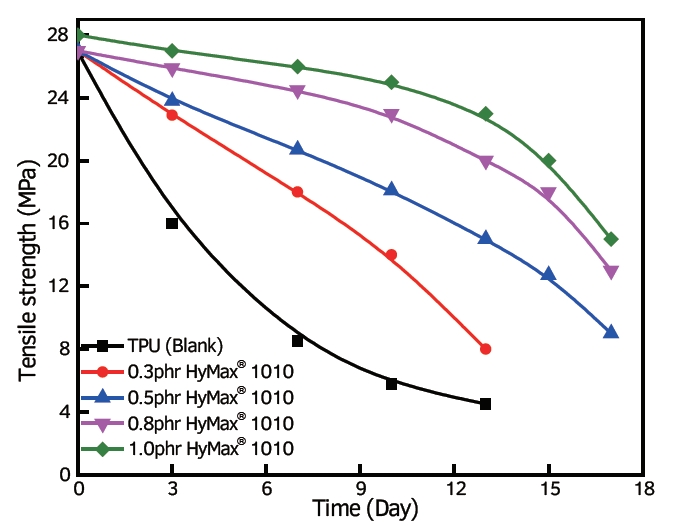
Anti-Hydrolysis agent in Polyester Polyurethane
Conclusion:
Understanding polymer hydrolysis, and the role of urethane in it, is essential for the production long-lasting polyurethane products. Anti-hydrolysis agents based on carbodiimide can be added to the mix to reduce hydrolysis. The materials will last longer and be more durable.