What is hydrolysis?
click to see more about hydrolysis
Does hydrolysis break apart polymers?
Yes, hydrolysis plays a significant role in breaking down polymers. Polymers, which are large molecules made up of repeating subunits, have covalent bonds between these units. Hydrolysis disrupts these bonds by inserting water molecules, causing the polymer chains to fragment into smaller components.
How does hydrolysis degrade polymers?
The process of hydrolysis involves the cleavage of covalent bonds within the polymer structure. Water molecules (H2O) participate in these reactions by substituting atoms or groups within the polymer chain. This substitution weakens the bonds, leading to the fragmentation of the polymer into smaller molecules or monomers.
Which polymers degrade by hydrolysis?
Various polymers are susceptible to degradation via hydrolysis. Polyesters, such as PET (polyethylene terephthalate) used in plastic bottles, and polyamides like nylon are examples of polymers that undergo hydrolytic degradation. Polysaccharides, including cellulose found in plant cell walls, are also subject to hydrolysis under specific conditions.
How to Solve the Hydrolysis Problem of Polymers
The susceptibility of polymers to hydrolysis can pose significant challenges in various industries, prompting the need for solutions to mitigate this degradation. Here are some strategies employed to address the hydrolysis problem in polymers:
1. Chemical Modification: Altering the chemical structure of polymers can enhance their resistance to hydrolysis. Introducing functional groups or additives that repel water molecules or strengthen the polymer’s backbone can reduce its susceptibility to hydrolytic degradation. For instance, incorporating hydrolysis stabilizers or anti-hydrolysis agents can bolster the polymer’s resistance to water-induced degradation.
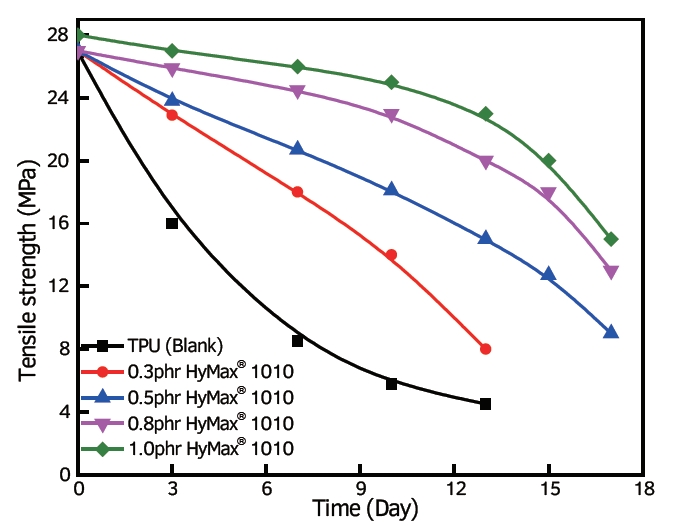
effect on TPU added with anti-hydrolysis agent
2. Barrier Coatings and Films: Applying protective coatings or films on the surface of polymers acts as a barrier against water penetration. These coatings, often composed of hydrophobic materials or specialized barrier polymers, shield the underlying polymer from direct contact with moisture, slowing down the hydrolysis process.
3. Environmental Control: Managing environmental conditions, such as temperature and humidity, can significantly impact a polymer’s susceptibility to hydrolysis. Storing or utilizing polymers in controlled environments with low humidity levels or stable temperatures can help mitigate hydrolytic degradation.
4. Selection of Resistant Polymers: Choosing polymers inherently resistant to hydrolysis for specific applications can be an effective preventive measure. For instance, certain engineering plastics like polypropylene (PP) or polyethylene (PE) demonstrate higher resistance to hydrolytic breakdown compared to other polymers.