In life, we often overlook the small but vital details. Take zippers for example, their existence and application have long been deeply rooted in our daily lives. In our clothes, shoes, hats, bags and even camping equipment, zipper is an important part, and sometimes it is even the key to measure the quality of a product. Not only is the durability of the zipper itself required, but also its comfort and aesthetics.
In many industries and applications, zippers need to work properly in high temperature and high humidity environments. For example, special protective clothing in outdoor sports equipment (e.g., camping tents, weatherproof jackets, etc.), industrial protective clothing, fire rescue equipment, and even aerospace equipment may need to operate in such environments. Considering that both the functionality and durability of zippers are critical in these settings, materials that can withstand high temperatures and high humidity need to be selected. And carbodiimide plays a vital role in this.
Hydrolysis of polyester materials:
Polyesters are a class of polymers in which repeating units are linked by ester bonds. Hydrolysis is the breaking of chemical bonds in the presence of water, which leads to the degradation of the molecule. For polyester materials, hydrolysis usually refers to the hydrolysis of the ester bonds. Hydrolysis of polyesters can be either a chemical reaction or an enzyme-catalyzed biodegradation.
See more about: what is hydrolysis?
The process of hydrolysis of polyesters can be simplified into the following steps:
Hydrolysis of the ester bond: In the presence of water, the ester bond undergoes a hydrolysis reaction that cuts the polymer molecule into smaller fragments.
Formation of carboxylic acids or alcohols: The products of hydrolysis are usually carboxylic acids and/or alcohols. These products have a higher water solubility, which promotes degradation and solubilization of the polyester material.
Degradation and decomposition: The carboxylic acids and alcohols resulting from hydrolysis can be further degraded and decomposed, ultimately releasing monomers or small molecule compounds.
Hydrolysis of polyester materials can be irreversible, meaning that once hydrolysis occurs, the structure of the polyester is destroyed and it is very difficult to return to its original polymerized state.
This is the reason why many polyester products lose their original properties under prolonged high temperature and high humidity.
How to improve the hydrolysis of polyester material and extend the service life of polyester zipper?
The following measures can be taken:
Choose suitable polyester materials: different types of polyester materials have different hydrolysis properties. Selecting a polyester type with higher hydrolysis resistance can extend the service life of the zipper.
Adding anti-hydrolysis agents: Adding anti-hydrolysis agents or antioxidants to the polyester material can slow down the hydrolysis reaction and thus extend the life of the material. Anti hydrolysis agents can react with carboxylic acids to repair broken molecular chains and block the hydrolysis reaction. This extends the life of the polyester material.
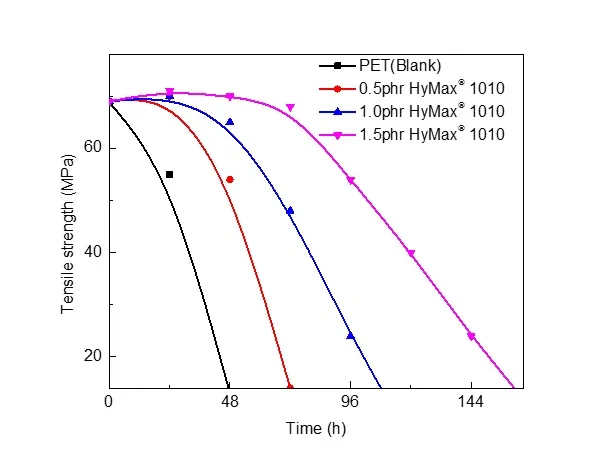
Testing effect of anti-hydrolysis agent in PET
Surface treatment: Through surface treatment methods, such as coating or plating, a protective film can be formed on the surface of the polyester material, blocking the entry of moisture and oxygen and reducing the occurrence of hydrolysis reactions.
Changing structural design: Optimizing the structural design of zippers, such as increasing sealing performance and reducing the presence of cracks and defects, can reduce the erosion of the material by moisture and oxygen and prolong its service life.
Apply appropriate lubricants: Using appropriate lubricants in zippers can reduce friction and wear and reduce the friction between zipper components, thus extending service life.
Avoid exposure to harsh environmental conditions: Try to avoid exposing polyester zippers to high temperature, high humidity or strong acid and alkali environments, as these conditions will accelerate the hydrolysis reaction.
Through the combined application of the above measures, the hydrolysis performance of polyester material can be effectively improved, and the service life of polyester zipper can be extended.
In general, the article underscores the importance of polyester zippers in various applications and highlights the challenge of hydrolysis in polyester materials, particularly in high temperature and humidity environments. Carbodiimide is proposed as a key solution to enhance the durability of polyester zippers. Strategies to mitigate hydrolysis and extend service life include selecting suitable materials, adding anti-hydrolysis agents, employing surface treatments, optimizing structural designs, using lubricants, and avoiding harsh environments. By integrating these measures, the hydrolysis resistance of polyester materials can be improved, thus extending the lifespan of polyester zippers.