What is hydrolysis in polyurethane?
Hydrolysis in polyurethane refers to a chemical reaction where the polyurethane material breaks down due to the presence of water or moisture. Polyurethane is a versatile polymer used in various applications due to its durability and flexibility. However, it is susceptible to hydrolysis, especially when exposed to water or high humidity for extended periods.
During hydrolysis, water molecules react with the chemical bonds in the polyurethane structure, causing them to break. This chemical breakdown can lead to several detrimental effects on the polyurethane material, including:
Reduced strength and mechanical properties: Hydrolysis weakens the molecular structure of polyurethane, compromising its strength, elasticity, and overall mechanical properties. This can result in deformation, cracking, or failure of the material.
Loss of flexibility and resilience: The degradation caused by hydrolysis can make polyurethane less flexible and resilient, affecting its ability to withstand stress or deformation.
Surface deterioration: Hydrolysis can manifest as surface degradation, causing the material to become rough, brittle, or discolored. This can significantly impact the appearance and functionality of polyurethane products.
Shortened lifespan: Materials undergoing hydrolysis tend to have a reduced lifespan, leading to premature failure or the need for replacement sooner than expected.
Why is PU hydrolysis?
Polyurethane (PU) is susceptible to hydrolysis due to the nature of its chemical structure and the presence of ester or urethane linkages within the polymer chains.
The hydrolysis of PU occurs because of the vulnerability of these linkages to reactions with water molecules. The ester or urethane groups within the polyurethane structure can be chemically cleaved when they come into contact with water or moisture. This chemical reaction involves the breaking of these bonds, leading to the degradation of the polymer chains.
The specific mechanisms can vary based on the type of polyurethane, its formulation, and the conditions of exposure to water or humidity. For example:
Ester hydrolysis: Some polyurethanes contain ester linkages within their structure, which are particularly susceptible to hydrolysis. When water molecules interact with these ester bonds, they undergo hydrolysis, causing the polyurethane chains to break down.
Urethane hydrolysis: Urethane linkages present in polyurethane can also be susceptible to hydrolysis under certain conditions. Water can react with the urethane bonds, leading to the cleavage of these linkages and contributing to the degradation of the material.
Factors such as temperature, pH, humidity levels, and the duration of exposure to water play crucial roles in accelerating or decelerating the hydrolysis process. Higher temperatures and increased moisture content typically expedite the degradation of polyurethane.
To combat hydrolysis, manufacturers often modify polyurethane formulations by incorporating additives, such as anti-hydrolysis agent (for solvent-based systems) or crosslinking agent (for water-based systems) or altering the chemical structure to enhance resistance to water or moisture. By improving the material’s resistance to hydrolysis, they can prolong its durability and usability in various applications where exposure to moisture is a concern.
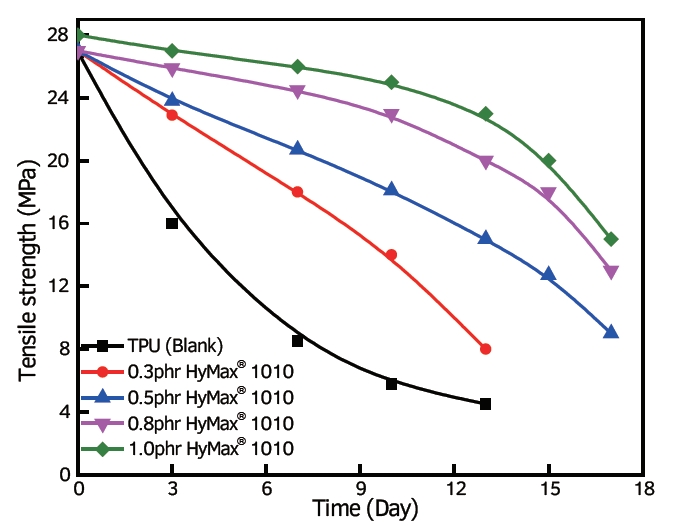
HyMax®anti-hydrolysis agent significantly increased the tensile strength of the TPU(80℃ boiled)
What is the hydrolysis test for polyurethane?
The hydrolysis test for polyurethane is a method used to evaluate the material’s resistance to degradation when exposed to water or moisture. This test helps assess the durability and stability of polyurethane formulations in environments where moisture ingress is a concern.
There isn’t a standardized hydrolysis test specifically designated for polyurethane; however, various methods and standards are employed by manufacturers or researchers to simulate hydrolytic degradation. One common approach involves subjecting polyurethane samples to accelerated aging conditions, replicating the effects of moisture over an extended period.
Here are general steps that may be involved in a hydrolysis test for polyurethane:
Sample preparation: Polyurethane samples are prepared according to the desired shape and size for testing.
Exposure to moisture: The samples are placed in an environment with controlled humidity or immersed in water to simulate prolonged exposure to moisture. The conditions can vary depending on the specific test setup and objectives.
Testing duration: The samples are kept under these conditions for a specified period, often for weeks or months, to observe any changes in the material properties.
Analysis: After the designated exposure period, the samples are removed, and various analyses are conducted to assess changes in properties such as mechanical strength, flexibility, appearance, chemical composition, and any other relevant characteristics.
Comparison: The results obtained from the exposed samples are compared to control samples that were not subjected to hydrolysis conditions. This comparison helps evaluate the extent of degradation and the material’s resistance to hydrolysis.
Data interpretation: Based on the observed changes, researchers or manufacturers can draw conclusions about the material’s susceptibility to hydrolysis and its potential performance in real-world conditions with moisture exposure.
The specific parameters, testing duration, and analysis methods used in a hydrolysis test can vary depending on the intended application, industry standards, or research objectives. These tests aid in understanding how different polyurethane formulations respond to moisture and help in developing more durable and moisture-resistant materials for specific applications.