Hydrolysis is a chemical reaction in which a substance reacts with water, leading to the breakdown of chemical bonds in that substance and the formation of new compounds. Hydrolysis typically involves the cleavage of larger molecules into smaller ones through the addition of water molecules.
There are different types:
Acid Hydrolysis: Strong acids break things apart.
Base Hydrolysis: Strong bases help break things apart.
Enzymatic Hydrolysis: Special chemicals called enzymes help break things apart, like breaking food into tiny pieces in your stomach.
Salt Hydrolysis: Sometimes, the salt you use in cooking can react with water and make things more acidic or basic.
Ester Hydrolysis: Water breaks apart esters, which are found in fats and other compounds.
Phosphoric Acid Ester Hydrolysis: Water helps break down compounds like ATP, which store energy in our cells.
Peptide Bond Hydrolysis: In our bodies, water helps break the bonds that hold proteins together.
These types of hydrolysis happen in various situations and are important in chemistry, biology, and everyday life. The main idea is that water is involved in breaking chemical bonds.
An anti-hydrolysis agent is a substance or chemical compound used to inhibit or prevent hydrolysis, which is a chemical reaction involving the breakdown of molecules due to the addition of water. Hydrolysis reactions typically involve the cleavage of chemical bonds with the incorporation of water molecules.

The hydrolysis of esters typically occurs through a reaction known as “acid-catalyzed ester hydrolysis” or “acidic hydrolysis of esters.” This reaction involves the cleavage of the ester bond, which is a covalent bond between the carbonyl carbon atom (C=O) of the ester and an oxygen atom (O) from an alcohol. Acidic hydrolysis of esters is commonly used in laboratories and in various chemical processes.
The overall reaction can be summarized as follows:
Ester + Water + Acid → Carboxylic Acid + Alcohol
Ester-based polymer materials are synthesized by the condensation of polyols and polyacids, such as PET, PBT, PU, PLA, PBAT, PC, and others. PET exhibits excellent fiber-forming properties, mechanical performance, wear resistance, creep resistance, and electrical insulation properties. PU offers high strength, wear resistance, and oil resistance. PLA, PBAT, and similar materials are biodegradable, environmentally friendly options.
However, ester-based polymer materials commonly have one drawback: they are prone to hydrolysis, which can reduce their lifespan.
HyMax anti-hydrolysis additives can react with carboxylic acids produced during polymer decomposition. This reaction forms stable urea group compounds without any adverse side effects. Consequently, it retards the hydrolysis of the polymer, ultimately extending the lifespan of the products.
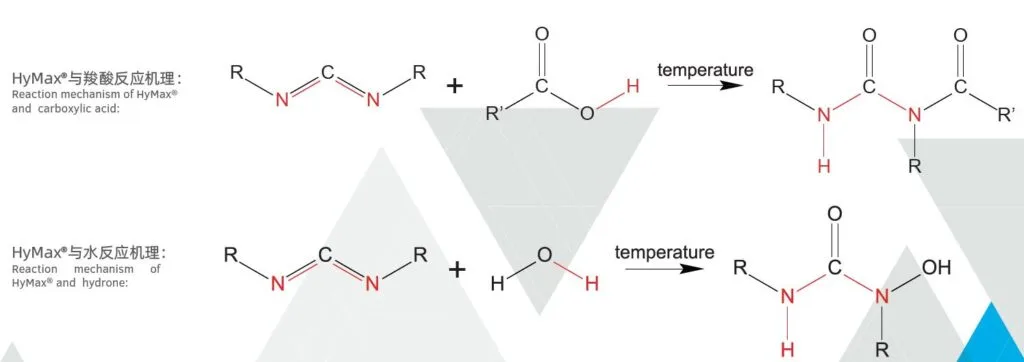
Anti-hydrolysis agents are used in various industries and applications to protect materials and substances from degradation or damage caused by hydrolysis. These agents work by either preventing the access of water molecules to the susceptible bonds or by stabilizing the bonds themselves.
In the context of specific industries and applications, anti-hydrolysis agents may go by different names or be referred to as stabilizers, inhibitors, or preservatives, depending on their function and purpose. In the manufacturing of plastics and polymers, anti-hydrolysis agents can be added to improve the material’s resistance to moisture and hydrolytic degradation. Here are the examples of application materials:
Please feel free to contact us. We will answer your questions in about 1 working day.
We will contact you within 1 working day, please pay attention to the email with the suffix “@langyitech.com”.