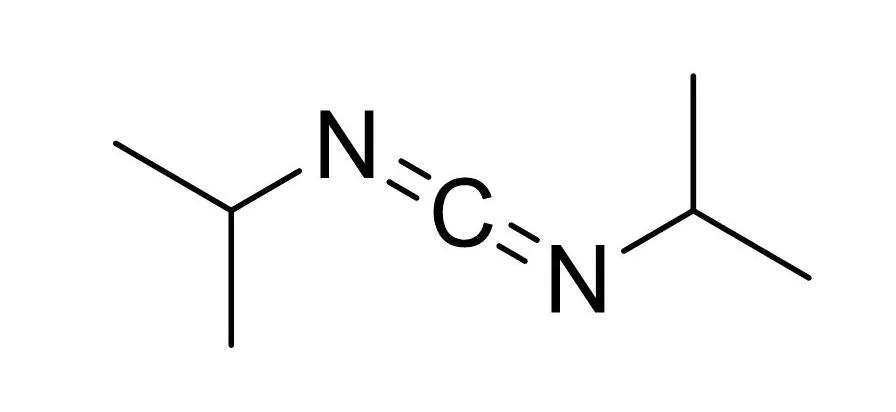
Carbodiimide, contains N=C=N functional groups, and is a commonly used dehydrating agent. It is generally prepared by thiourea losing hydrogen sulfide or urea losing water, and hydrolyzed to obtain urea derivatives. Mainly used to activate carboxyl groups to promote the formation of amides and esters.
Basic Information of carbodiimide
English name: Carbodiimide
Alias: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
IUPAC name: 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride
Identification
CAS number 25952-53-8
SMILES CCN=C=NCCCN(C)C
Nature
Chemical formula: C8H17N3·HCl
Molar mass: 191.70 gmol-1
Application of carbodiimide
In organic synthesis, carbodiimide is a commonly used water loss agent, mainly used to activate carboxyl groups to promote the formation of amides and esters. N-hydroxybenzotriazole or N-hydroxysuccinimide is often added in the reaction, which can increase the yield and reduce the occurrence of side reactions.
Carbodiimide can also react with amines to form guanidine.
Mechanism
The carboxylic acid first reacts with the carbodiimide to form the intermediate O-acyl isothiourea, similar to the introduction of an ester group to activate the carboxylic acid. Then the O-acyl isothiourea reacts with the amine to form the target product amide and urea. O-acyl isothiourea can react with another molecule of carboxylic acid to generate acid anhydride, and the reaction of acid anhydride with amine can also generate amide.
The by-product of the reaction is mainly N-acylurea formed by the rearrangement of O-acylisothiourea. The use of low dielectric constant solvents (such as dichloromethane, chloroform) can reduce the formation of N-acylurea.
Examples of carbodiimide
HyMax 1010
Synonyms: Carbod; staboxol1;Stabilizer 7000; RARECHEM AQ A4 0133; Bis(2,6-diisopropylp; STABILIZER 7000 / 7000F; (2,6-diisopropylphenyl)carbodiimide; bis(2,6-diisopropylphenyl)-carbodiimid;N,N’-Bis(2,6-diisopropylphenyl)carbodiimide
Molecular Formula: C25H34N2
CAS Number:2162-74-5
Specification:
Appearance: White to pale yellow crystalline powder
Assay: ≥98 %
Melting Point: 49-54°C
Applications :
- It is an important stabilizer of polyester products (including PET, PBT, and PEEE) ,TPU,PA, PC, polyurethane products, polyamide nylon products, and degradable plastics such as PLA,EVA.
- It can prevent water and acid attacks to grease and lubricating oil, enhance the stability .
- It can improve the hydrolysis resistance stability performance and extend the service life of many polymers,including PU, PET, PBT, TPU, CPU, TPEE, PA6, PA66, EVA and so on, especially at high temperature under the condition of damp, acid and alkali.
- It can prevent the loss of polymer molecular weight in the production process
DCC
DCC (dicyclohexyl carbodiimide) is one of the earliest used carbodiimides, and it is mostly used in the peptide synthesis step. The reaction yield of DCC as a dehydrating agent is very high, and the price of the reagent is not expensive.
Physical and chemical properties:
Chemical formula: C13H22N2
Molecular weight: 206.1
CAS Registry Number: 538-75-0
Melting point: 33-35°C
Boiling point: 122-124°C
Density: 1.325g/cm^3
Appearance: white or light yellow crystal
Flash point: 87°C
However, DCC also has many shortcomings that limit its application:
1. The by-product N,N’-dicyclohexylurea is insoluble in water and is generally removed by filtration, but there is still a small amount of residue in the solution, which is difficult to remove;
2. DCC is not as convenient as other solid-phase peptide reagents, and the product dicyclohexylurea is difficult to separate from the peptide resin;
3. DCC can cause allergies.
DIC
DIC (N,N’-diisopropylcarbodiimide) is used as a substitute for DCC.
Physical and chemical properties:
Properties: colorless transparent liquid
Appearance: colorless to light yellow liquid
Main content: ≥99.0%
Boiling point: 145-148 °C
Density: 0.815 g/mL at 20 °C
Flash point: 93°C
Sensitivity: Water absorption
Dangerous goods signs: T+, T, F
Hazard category code: 10-26-36/37/38-41-42/43
Safety instructions: 26-36/37/39-45-38-28A-16
Un code: UN 2929 6.1/PG 1
Uses: Mainly used as a condensing agent in peptide synthesis.
Compared with DCC, it has the following advantages:
1. DIC is liquid and easier to use;
2. The product N,N’-diisopropylurea is soluble in most organic solvents and can be easily removed by solvent extraction. DIC is therefore commonly used in solid-state synthesis;
3. DIC is less likely to cause allergies.
EDC
EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride) is a water-soluble carbodiimide. It is used as a carboxyl activator in the synthesis of amides. It is also used For the preparation of activated phosphate groups, cross-linking of proteins and nucleic acids and immunoconjugates. The pH range during use is 4.0-6.0, and it is often used in conjunction with N-hydroxysuccinimide (NHS) or N-hydroxysulfosuccinimide to improve coupling efficiency. In organic chemistry, EDC and the catalyst 4-dimethylaminopyridine (DMAP) are used to esterify carboxylic acids and alcohols.
Physical and chemical properties:
Melting point: 110-115 °C
Density: 0.877 g/mL at 20 °C(lit.)
Refractive index: n20/D 1.461
Storage conditions: −20°C
Solubility: soluble in water.